Arrange These Bonds From Most Polar to Least Polar
When you do a chemical reaction this is the amount of chemical that you actually make ie. This refers to a condition in which an area has at least 20 per cent households facing extreme lack of food.
Polarity Of Molecule Exam Question
Why do objects emit electromagnetic radiation EMR in a range of wavelengths.

. Neopentane molecules are the most compact of the three offering the least available surface area for intermolecular contact and hence the weakest dispersion forces. Place the shortest wavelength at the bottom Not all molecules in an object vibrate or move at the same speed. But many weak bonds can act in parallel to hold two regions of a polypeptide chain tightly together.
The ability of carbon to form chains gives rise to a homologous series of compounds in which the same functional group is attached to carbon chains of different lengths. Figure PageIndex4 compares the electron distribution in a polar covalent bond with those in an ideally covalent and an ideally ionic bond. These carbon chains may be in the form of straight chains branched chains or rings.
The majority of our activities including all public events are still online. The atomic weight of an element is the weighted average of the mass of all the _____ of a particular element. The amount of stuff you can weigh.
This is when you arrange elements in the order of how much they tend to react with water and acids. The Royal Societys building is open in a limited capacity for some specific events and pre-booked venue hire. The second shell has two kinds of orbitals _____.
Each of these orbitals has a unique shape and occupies a defined space. IPC defined famine as an extreme deprivation of food. The weak bonds are of three types.
The first shell has one orbital called an _____ orbital. The least electronegative atom must be considered the central atom since it has the highest ability to share its electrons with other atoms belonging to the molecule. Hydrogen bonds ionic bonds and van der Waals attractions as explained in Chapter 2 see p.
A share of the population in these four countries were projected to experience starvation and death in the last assessment by the agencies for August to November 2021. A reaction where atoms add to a. The stability of each folded.
The measure of how easy or difficult it is for another electrostatic charge for example a nearby ion or polar molecule. Individual noncovalent bonds are 30300 times weaker than the typical covalent bonds that create biological molecules. The minor isotope of hydrogen that contains one proton and one neutron in.
Facilitated diffusion proteins shield these materials from the repulsive force of the membrane allowing them to diffuse into the cell. An object with an intermediate temperature. Carbon also forms compounds containing double and triple bonds between carbon atoms.
Now what BHT and its metabolies do is to very much disrupt these hydrogen bonds and thereby denature at least some proteins in certain protein coated viruses enough to destroy at least some proteine coated viruses including at least. Arrange the parts of the electromagnetic spectrum listed below by wavelength. However these materials are ions or polar molecules that are repelled by the hydrophobic parts of the cell membrane.
Then Count the total number of electrons belonging to the outermost shell of the central atom. The covalent bonds are NOT broken and yet these proteins collapse because these hydrogen bonds disappear in a non polar liquid. These proteins are called transportproteins and can be channels or carrier proteins.
Bonds still vibrate a little bit but for the most part you dont see much happening. Arrange the following compounds from highest boiling point to lowest boiling point and explain your answer on the basis of whether the substance is. Recall that a lowercase Greek delta delta is used to indicate that a bonded atom.
SECONDARY STRUCTURE 2 structures Polypeptide chain can arrange itself into characteristic helical or pleated segments Given by Pauling and Corey hydrogen bonding interactions between adjacent amino acid residues Free rotation is possible about only two of the three covalent bonds of the polypeptide backbone α-carbon Cα to the carbonyl carbon. Most compounds however have polar covalent bonds which means that electrons are shared unequally between the bonded atoms. Count the total number of electrons belonging to the other atoms and use them in bonds with the central atom.
This behavior is analogous to the connections that may be formed between strips of VELCRO.

Bond Polarity Chemistry Lessons Chemistry Education Organic Chemistry Study

Is Pbr5 Polar Or Nonpolar Phosphorus Pentabromide Chemical Formula Phosphorus Molecules
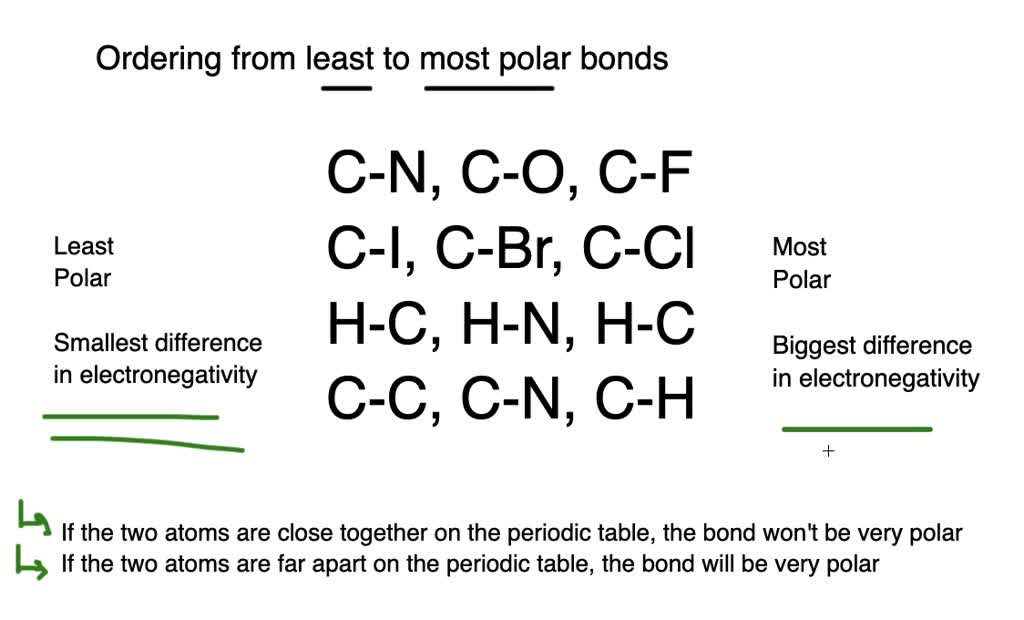
Solved Rank The Bonds From Most Polar To Least Polar A Quad Mathrm C Mathrm O Mathrm C Mathrm F Mathrm C Mathrm N B Quad Mathrm C Mathrm Cl Mathrm C Mathrm I Mathrm C Mathrm Br C Quad Mathrm H Mathrm O Mathrm H

Is H2o Polar Or Nonpolar Water Polarity Of Water Polar Molecules

Oneclass The Four Bonds Of Carbon Tetrachloride Cci 4 Are Polar But The Molecule Is Nonpolar Beca
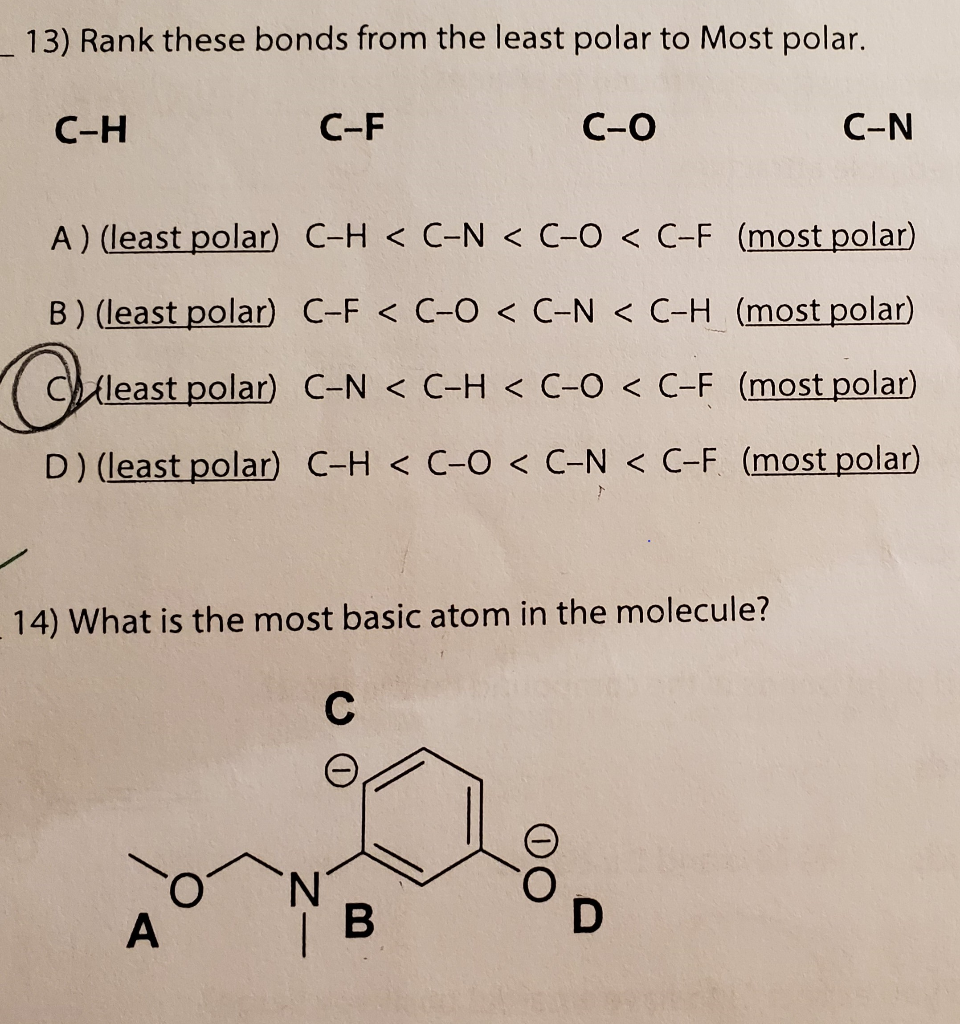
Solved L 13 Rank These Bonds From The Least Polar To Most Chegg Com
Rank The Bonds From Most Polar To Least Polar A C O C F C N B C Ci C I C Br C H O H N H C D C H C C C N Study Com


Comments
Post a Comment